Materials:
Spinach leaves
Blender
Beaker
Rubbing alcohol
Gauze
Black light
First me, sam, and josh grabbed a handful of spinach leaves and put them into a blender. After we poured enough of rubbing alcohol into the blender so that the alcohol covered the leaves. Completely blending the materials together, they turned out to turn the clear liquid into a green color. Next we took the gauze and covered the beaker so that no liquid could go through with out entering through the gauze first. Taking the blender with the contents inside slowly poured the liquid and spinach into the beaker. Once all the liquid was poured all the gauze filtered the solids outs. Now that the chunkier parts are out we placed it under the black light. Why did it glow red? The liquid was green in regular light.
When the florescent light is directed toward the chlorophyll and thats when the chlorophyll electron become excited. Because the chloroplast thylakoid membranes were dissolved there is no cytochrome transport system. The chlorophyll absorbed the light and give up their energy state which releases its reddish glow.
Wednesday, December 21, 2011
Tuesday, December 20, 2011
Cell membrane structure
In the picture you see above is all the parts of the cell membrane.
The cell membrane is the outer part of the cell that consists of a lipid ilayer with proteins embedded in it.
Phospholipid Bilayer
 This area, the Phospholipid Bilayer sets the boundaries of a cell. The Phospholipid is composed of two lays of fat cells. Inside this layer it consists of:
This area, the Phospholipid Bilayer sets the boundaries of a cell. The Phospholipid is composed of two lays of fat cells. Inside this layer it consists of:
The cell membrane is the outer part of the cell that consists of a lipid ilayer with proteins embedded in it.
Phospholipid Bilayer
 This area, the Phospholipid Bilayer sets the boundaries of a cell. The Phospholipid is composed of two lays of fat cells. Inside this layer it consists of:
This area, the Phospholipid Bilayer sets the boundaries of a cell. The Phospholipid is composed of two lays of fat cells. Inside this layer it consists of: The hydrophilic polar head which likes water and associates with water that is outside the cell. There is a positive charge towards the water and negative charges away from the water. The phospholipid molecule's polar head group contains a phosphate group.
The Hydrophobic non-polar tails don't like water. These tails are fatty acids.
Thes tails are a non-polar fat acid.
Integral Proteins
These types of proteins are embedded into the bilayer of the membrane. The integral protein floats freely throughout the bilayer and turns into a transmembrane protein because it extends through the lipid bilayer. Each end comes into contact with the exterior. The integral protien is hydrophobic and made up of non-polar amino acids and the soon exposed ends of the integral protien are hydrophilic.
Other examples of integral membrane proteins are:
glucose premease
hormone receptors
ion channels and gates
Peripheral Proteins
Friday, December 16, 2011
Black Widow
 Black widows have shiney black body and measure to one-half inch to an inch in body length. A female has a marking the color of orange-red shaped hour glass on its belly. Males on the other had have more of a red hourglass marking but much smaller. A male widow is harmless, to where as a female is very venomus. It injects the victim with venom which is neurotoxins, then puts digestive juice which decomposes the skin. It makes it easier for the widow to feed, then sucks out the digestive juice Facts:
Black widows have shiney black body and measure to one-half inch to an inch in body length. A female has a marking the color of orange-red shaped hour glass on its belly. Males on the other had have more of a red hourglass marking but much smaller. A male widow is harmless, to where as a female is very venomus. It injects the victim with venom which is neurotoxins, then puts digestive juice which decomposes the skin. It makes it easier for the widow to feed, then sucks out the digestive juice Facts:-ledges, rocks, plants and debris, any place an acutally web can be. In the winter it can force spiders into buildings, home, etc..(habitat)
-black widows prey on insects by catching them inside their web.
-its preyed upon MUD-DAUBER Wasp
-”Latrodectus” is another name for a Black widow
 -As newly hatched black widows they are white with black spots on the abdomen and have a cream colored hourglass.
-As newly hatched black widows they are white with black spots on the abdomen and have a cream colored hourglass. -Females after a two year life span they produce several egg sacs
Thursday, December 15, 2011
Enzyme Action Labs
Enzyme(# of yeast drops)
To start off, my class played a game to try and get a feel for what an enzyme action does. By having the enzymes chase the glucose and capture it each time and then later adding inhibitors and enzyme the less glucose there was.Lab Group: Brett, Me, Josh, and Sam
 We later started a lab in which we'd be testing the amount of pressure the yeast had on the other materials in the test tube. Our first lab we started by adding 3ml of 3% H2O2 and 3ml of water in to 3 different test tubes. To not mess up our lab results we would conduct each separately. We first added 10 drops of the mixed yeast into test tube one and put the gas pressure sensor inside the tube. 3 minutes of the pressure would be measured each tube and our computer would keep track of the rising pressure of the gas. Once 3 minutes had passed we took of the gas pressure sensor and received 8.007 kPa for 10 drops. After taking of the gas pressure sensor we added 15 yeast mix drops into the 2nd test tube. After putting on the gas pressure sensor on our second test tube we let the 3 minutes pass and let the computer record our data.Our data showed that the 15 drops of yeast had the pressure of 11.4 kPa. Finally we'd be test 20 drops of yeast mix. 20 drops was put into the 3rd test tube and had the gas pressure sensor put into it. 3 minutes of watching the line incline the data showed 8.583 kPa. My bar graph shows that the pressure was highest when there was 15 drops of yeast put into the H2O2 and water. The tube with only 10 drops came out with a pressure of 8.007 kPa, which is the lowest pressure in this experiment.
We later started a lab in which we'd be testing the amount of pressure the yeast had on the other materials in the test tube. Our first lab we started by adding 3ml of 3% H2O2 and 3ml of water in to 3 different test tubes. To not mess up our lab results we would conduct each separately. We first added 10 drops of the mixed yeast into test tube one and put the gas pressure sensor inside the tube. 3 minutes of the pressure would be measured each tube and our computer would keep track of the rising pressure of the gas. Once 3 minutes had passed we took of the gas pressure sensor and received 8.007 kPa for 10 drops. After taking of the gas pressure sensor we added 15 yeast mix drops into the 2nd test tube. After putting on the gas pressure sensor on our second test tube we let the 3 minutes pass and let the computer record our data.Our data showed that the 15 drops of yeast had the pressure of 11.4 kPa. Finally we'd be test 20 drops of yeast mix. 20 drops was put into the 3rd test tube and had the gas pressure sensor put into it. 3 minutes of watching the line incline the data showed 8.583 kPa. My bar graph shows that the pressure was highest when there was 15 drops of yeast put into the H2O2 and water. The tube with only 10 drops came out with a pressure of 8.007 kPa, which is the lowest pressure in this experiment.Enzyme( temperature)
 The next day we tested the pressure on 20 drops of yeast in different types of temperature. We first filled 4 test tubes with H2O2 and water then placed each tube into a different temperate area. Placing one in ice, one in hot water, once at room temperature, and in warm water. The most reasonable part would be to start with the room temperature because we don't have to wait for the water to heat up or cool down. So we added 20 drops of yeast and then put the gas pressure sensor on and measured its pressure for 3 minutes. Room temperature shows 8.587 kPa. After about 5-10 minutes we recieved the cold water and added its 20 drops of yeast and started to measure its gas temperature. 3 minutes later it showed 6.037 kPa. Next was the warm water. Adding yeast then letting the pressure of the gas be collected into the computer, we then came out with 7.427 kPa. Finally, hot water and 20 yeast drops was tested for the pressure it had and was recorded onto the computer. The hot water's pressure came out to 7.427 kPa. According to my results, the room temperature had the highest pressure than the cold, warm, and hot water.
The next day we tested the pressure on 20 drops of yeast in different types of temperature. We first filled 4 test tubes with H2O2 and water then placed each tube into a different temperate area. Placing one in ice, one in hot water, once at room temperature, and in warm water. The most reasonable part would be to start with the room temperature because we don't have to wait for the water to heat up or cool down. So we added 20 drops of yeast and then put the gas pressure sensor on and measured its pressure for 3 minutes. Room temperature shows 8.587 kPa. After about 5-10 minutes we recieved the cold water and added its 20 drops of yeast and started to measure its gas temperature. 3 minutes later it showed 6.037 kPa. Next was the warm water. Adding yeast then letting the pressure of the gas be collected into the computer, we then came out with 7.427 kPa. Finally, hot water and 20 yeast drops was tested for the pressure it had and was recorded onto the computer. The hot water's pressure came out to 7.427 kPa. According to my results, the room temperature had the highest pressure than the cold, warm, and hot water.Enzyme( Ph)
This time in the lab we will be testing the pressure of the Ph and yeast together. In each of the test tubes we added H2O2 and then the Ph. First we did the neutral ph and the pressure wiht the gas pressure sensor. After the 3 minutes had passed we collected our data. The neutral turned out with a pressure of 6.4 kPa. Repeating this same step for the acidic and basic Ph, the acidic ph pressure came out as 5.316 kpa and the basic ph pressure was 6.805.Analyzing the data collect, it showed that the basic ph had the highest pressure of the experiment with a range of 1.489 between the highes basic ph(6.805 kpa) and the lowest acidic(5.316 kPa).
Thursday, December 1, 2011
PKU
PKU occurs from an inherited genetic mutation that causes disruption of the PAH enzyme.
2. What reaction does this enzyme catalyze? (What is the substrate and what product is produced?)
Answer:
3. Describe the symptoms of phenylketonuria.
Answer:
4. What causes the symptoms of PKU, the lack of a substance or the buildup of one?
Answer:
PKU affects the ability to use protein properly. Enzymes break down proteins into amino acids tas building blocks for body growth and repair. Only in PKU the enzymes dont function properly. The one that is needed to convert "phe" into another amino acid. As a result to this, "phe" assembles in the blood and the body.
5. How common is phenylketonuria? How is it treated?
Answer:
PKU occurs in 1 in 10,000 to 1 in 15,000 newborns. Although this is so, the incidence varies according to ethnic groups and geographic location.
http://www.ygyh.org/pku/whatisit.htm
2. What reaction does this enzyme catalyze? (What is the substrate and what product is produced?)
Answer:
3. Describe the symptoms of phenylketonuria.
Answer:
Excess "phe" prevents growth in the brain and developing normally. Also skin rashes, musty body odor, metal retardation, and epilepsey.
4. What causes the symptoms of PKU, the lack of a substance or the buildup of one?
Answer:
PKU affects the ability to use protein properly. Enzymes break down proteins into amino acids tas building blocks for body growth and repair. Only in PKU the enzymes dont function properly. The one that is needed to convert "phe" into another amino acid. As a result to this, "phe" assembles in the blood and the body.
5. How common is phenylketonuria? How is it treated?
Answer:
PKU occurs in 1 in 10,000 to 1 in 15,000 newborns. Although this is so, the incidence varies according to ethnic groups and geographic location.
http://www.ygyh.org/pku/whatisit.htm
Tuesday, November 29, 2011
Cystic Fibrosis
The site where I recieved my information is of following
http://www.cff.org/AboutCF/
1. What are the signs and symptoms of cystic fibrosis?
The symptoms of Cystic fibrosis are salty-tasting skin, persistent coughing, frequent lung infections, shortness of breath or wheezing, poor growth, weight gain, and/or bulky stools.
2. How common is this disorder?
In about 1,000 cases of Cystic fibrosis are diagnosed every year. More than 45% of the Cystic fibrosis patient population is age 18 and up. Over 70% of patients diagnosed are by the age of two. The median age of a survival for a person that has CF is in the late 30’s.
3. How is cystic fibrosis diagnosed?
Cystic fibrosis is diagnosed by the clogging of the lungs that leads to life-threatening lung infections. Also obstruction to the pancreas that stops natural enzymes from breaking down or absorbing food in the body.
4. How is cystic fibrosis inherited? Does everyone who has a mutant gene for the protein have cystic fibrosis?
Cystic fibrosis is an inherited chronic disease affecting the lungs and digestive system of 30,000 children and adults in the U.S. (70,000 worldwide) 10 million Americans are carriers of the defective gene that show no symptoms the having CF. The product of the defective gene and the product cause the body to produce think sticky mucus.
http://resources.schoolscience.co.uk/MRC/3/page3.html
1. Explain the normal function of the protein that is defective in cystic fibrosis.
The CF transmembrane regulator ( CFTR) protein makes the chloride channel. This proteins normal function controls the flow of chloride ions from the cell.
2. What happens to this protein in CF patients and what are the consequences for the health of these individuals?
A person with CF has the cital chloride channel blocked. There is no movement of chloride ions into the mucus and the mucus starts to dry out.
For the questions below this is the link to where I received all of my information.
http://www.mayoclinic.com/health/cystic-fibrosis/DS002871. Explain at least 3 treatments for the symptoms of cystic fibrosis.
Three treatments for CF are Antibiotics, Mucus-thinning drugs, and therapy.
The Antibiotics are the drugs that help prevent lungs infections. These can be swalloed in pill form, inhaled in mist or delivered intravenously. Mucus-thinning drugs reduce the stickiness of the mucus to it makes coughing it up easier, helping the lung function properly.Therapy helps physically remove the think mucus from the lungs. By clapping on the back and front chest with cupped hands also helps get it out.

2. Discuss at least 3 ways for parents to help their children who have cystic fibrosis.
Parents that have children with cystic fibrosis should have their child exercise, drink lots of fluids, and keep immunizations up to date.
Exercise helps loosen mucus that is in the airways and heps strengthen the heart and lungs. Anything that gets the child moving would help loosen up that mucus.
Keeping immunizations up to date such as the influenza vaccines helps because although CF doesn’t affect the immune system the child still is more likely to develope complications when becoming sick.
Wednesday, November 9, 2011
Osmosis experiment(:
At first me, Josh, and Sam had to come up with a lab experiment that shows osmosis. The supplies given to us where cups, baggies, and a scale that measured weight in grams. We all came to a conclusion of how we were gonna conduct this experiment. First we gathered all of our experiment materials and placed them in our lab area. The experiment had to be controlled, so we filled our 3 cups with 5 oz. of water and selected our three baggies to put our substances into. Opening the top of the baggy I tied the bottom of the bag with about 5 inches of string so that our inside substances wouldn't come out the bottom. After all 3 baggies were tied we filled each with water, but not too much water to where we couldn't add anything into it. Now it was time to add our substances into the bags of water. 3 different types of products we were gonna test. Equate Antacids, Cheerios, and Light Corn Syrup we chosen to use. Crushing the 3 antacids, I had to make sure that the pieces were small enough to fit and cause osmosis. When it was finally crushed, Sam held open the bag and I poured the entire substance into the baggy. Finally, I tied the top of the bag and we set it aside. We followed the same steps with the cheerios. From crushing up 4 cheerios, to adding it into the water and tying the top. This time if the corn syrup we just had to pour some corn syrup into the baggie. After tying that last baggy, we grabbed all baggies with the substances in it, and weighed each separately. The baggy with the antacid results showed 12.88 grams. The baggy with the cheerios weighed 10.78. Next we weighed the baggy with the corn syrup showing 5.86. After all three were weighed we dropped them into the 3 seperate cups we started with. Our times to analyze our data was in 1 hour and a day later. An hour later I came in and took out the 3 baggies and dried them all. Once they were dried I took each and placed it on the scale one at a time and recorded its grams. Antacids pulled in 1.11 oz and now weighing 13. 99 oz. Cheerios pulled in .97 oz now weighing 11. 71 oz. Corn syrup gained .73 oz now weighing. The following day we then measured their weight once more. Antacids started with 12. 88 then an hour later weighed 13.99 and a day later weighed 13.55. Cheerios started with 10.78 then 11.71 and ended with 11.59. Corn syrup started with 5.86 then an hour later weighed 6.59 and ended with 5.95. My results showed that with their starting weighted osmosis allowed water in the hour before I collected my results. A day later osmosis occurred when letting water out because each weighed less than the hour later analysis.
By definition, osmosis is the tendency of a fluid, usually water to pass through asemipremeable membrane into a solution where the solvent concentration in higher, thus equalizing the concentrations of materials on either side of the membrane. In our lab osmosis occurred because water passed through the baggy from the outside inside of the baggy. Where the concentration is higher the water goes into. Connceting this to my lab, the water passed into the baggy because the concentration was higher and making it weigh more than it did before.
Sunday, October 2, 2011
PH Lab
In this lab me and megan both selected 4 antacids to mix in vinegar. We then filled 4 cups with 25mL of vinegar. Next we took we picked out 4 different types of antacids to test in the vinegar. First we took the PH number of vinegar to see which antacid works best. Vinegar showed a PH number of 2 to start with. Next we started to add our antacids into the cups. Peppermint Antacid with the lowest milligrams was crushed into powder and mixed with the vinegar. Once mixed we took its PH number the the PH tape. Our results showed that the PH number is 5 because we compared its color to the key on the box. Next we took the Equate regular antacid and once again crushed it into powered to mix with the vinegar. With the PH tape we took its PH number getting a 4. Rolaids had the next lowest dosage and so we crushed it and poured its powered into the vinegar filled cup. Mixed and tested, we came out with a PH number of 6. Finally Extra Rolaids Antacid had the highest dosage number and it was time to test it. Once it was mixed with the vinegar we took its PH number getting a 5. The results show the lowest dosage of Peppermint antacids and the highest dosage of Extra Rolaids both showed the same PH Number, 5. The Equate Regulars showed 4 with a lower PH number and Rolaids with a 6 being the highest PH number received in the entire experiment. Although both the lowest and highest dosage of antacids showed the same results, meaning they work the exact same the Rolaids having a PH number of 6 shows it works better than all the other antacids used in the experiment.
Thursday, September 22, 2011
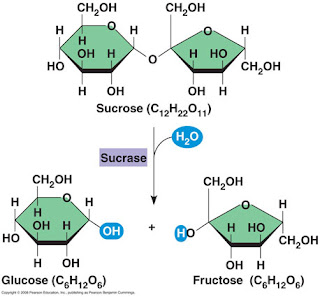
Carbs
1. What is a Macromolecule?
A macromolecule isa molecule containt a very large number of atoms. Also a class of large molecules.
2. What is a monomer?
A small molecule that is a subunits of a polymer.
3. What is a ploymer?
It's the largest macromolecule because they are linked together by large numbers of subunits called momomers.
4. List the 4 main types of Macromolecules?
Type Example
Proteins Polypeptide
Lipids Fat
Carbohydrates Polysaccharide
Nucleic Acids DNA, RNA
5. What are the types of reactions that Macromolecules are shown to undergo?
Macromolecules under hydrolysis reactions and and condensation reactions.
6. Describe how monomers are joined together.
Momomers are joined by dehydration synthesis.A covalent bond is formed between 2 monomers while water is formed by the OH groups.
7. Describe now polymers are broken down.
Polymers are broken down by hydrolysis reactions. A water molecule with the help of a hydrolase enzyme breaks the covalent bond that is holding the monomers together.
8. What's the specific name for the bond between simple sugar monomers?
A covalent bond.
9. Which kind of enzyme joins monomers together?
The hydrolase enzyme is what joins monomers together.
10. Describe how you had to arrange the sugar monomers in order to build a polysaccharide.
First I added a sugar molecule to another sugar molecule to form a glycosidic linkage. Next I add another sugar molecule to the other two sugar molecules. The oxygen molecules hold the sugar molecules together when i rotated it. I rotated it to separate the hydrogen molecules so that once the oxygen molecules where connected H2O was formed. Once all 4 sugar molecules where linked the bonds where held together and I created a 4-molecule polysaccharide.
11. Which building blocks of macromolecules are not used in building carbohydrates?
Amino acids, fatty acids, and nucleotide are not used to build a carbohydrate.
12. Why is sugar stored as glycogen in the human body?
Glycogen is stored in the human body. Glycogen is stored in the muscles and once its stored in the muscles it can't be released. Also it's stored in the liver because the liver helps regulate the amount of sugar goes into the blood stream. This balances out our blood sugar levels.
13. Why are plant foods essential in animal life?
Glucose is the fuel in all living things. Plants produce sugar during there living process. Our bodies have a hard time producing glucose and by eating vegetables and fruits we take in all of that glucose to store.
14. Describe how starch is digested by animals.
The starch first starts to digest first in the mouth with salivary amylase. It goes into the small intestines digesting in pancreatic amylase. Glucoamylase breaks the short chains of alpha-dextrin(glucoses) into individual glucose molecules that are then absorbed.
15. What is fiber and why is it important in your diet?
Fiber is filament formed from vegetable tissue, mineral substance, or textile. Fiber is important in our diet because it reduces the risk of cancers, diabetes, digestive disorders and heart disease.
16. What causes you to pass gas(fart) according to the article?
Indigestable carbohydrates produce gas.
17.disadvantages to a low-carb diet.
The disadvantages are that in the first few months there is a great weight loss but in the 12 months both energy and low-carb diets have the same results. Also you don't get enough carbs.
18. Sugars and cavities formation
Acid-producer+Carbohydrates+teeth= Cavities
When you eating sugars it produces acids that cause cavities.
All About Water
We use water in everything we do such as cooking, hygiene, swimming, drinking, watering plants, and even fun activities. Organisms are over 80% water and waters properties and the way we use it is very important. For every oxygen atom there is two hydrogen atoms. Oxgyen is short two electron in its outer shell and hydrogen is short one electron in its single shell and by sharing electrons they complete each others shell and make water.
In class we did an experiment where we would drop water drop by drop onto penny to see how many drops of water would stay on the penny before its bond broke. The water was very adhesive to the penny because it stuck to it well. On the wax paper the water wasn't as adhesive because you could easily move it around. Water is cohesive because although water flows freely the water molecules don't separate. Surface tension allows the water to attract to one another. Its lowest energy state surrounds the area around the molecule and as gravity acts on it it gives us the shape of the sphere that we see.
Water has high surface tension because of its hydrogen bonding. Water striders, insects walking on the surface of water, humans skipping rocks are able to do all this because of this property.
Friday, September 2, 2011
In text books the scientific method is simplified into five easy steps but in reality it's not that simple. Scientists work in different sequences and repeat the same step for accuracy and new ideas. In order to experiment scientists need an independent variable and dependent variable. An independent variable is the factor being changed in the experiment. In the corn experiment the types of corn or the amount of bugs you added to the one type of corn. The dependent variable is what you measured in the experiment. During the corn experiment the dependent variable was the corn growth in one-hundred and four days. The growth was effected by the infestation on both experiences.
Placebo is a pill that is an inactive medication. It is given somehow to the person and they mentally believe they are getting better, but their mind is making itself better. Any effect in improvement of health is called the placebo effect. In some trials, placebo can be given to a group rather then a drug. It's like the enthusiasm of the patients new drug, could help with the excitement they could get better.
2 dif corn...independant variable..how they grow is dependent
Placebo is a pill that is an inactive medication. It is given somehow to the person and they mentally believe they are getting better, but their mind is making itself better. Any effect in improvement of health is called the placebo effect. In some trials, placebo can be given to a group rather then a drug. It's like the enthusiasm of the patients new drug, could help with the excitement they could get better.
2 dif corn...independant variable..how they grow is dependent
Subscribe to:
Posts (Atom)